Bakground:
Preeclampsia occurs in up to 7% of pregnancies in Western Countries and is a leading cause of maternal and fetal morbidity and mortality. Classification of preeclampsia is based on the timing of symptoms' onset and the severity of the clinical status. Accordingly, early and late preeclampsia are characterized by symptom onset before or after 32-34 weeks of gestation respectively. Pathogenesis of preeclampsia is characterized by abnormal placental vascular development with impaired invasion of the uterine spiral arteries by trophoblasts, resulting in fetal complications and maternal endothelial dysfunction. Intact antithrombotic properties of the endothelial cells and efficient regulation of thrombin generation and platelet activation at the microenvironment of the uterus are necessary conditions for successful trophoblast invasion and development of feto-maternal circulation during normal pregnancy. , Prompt identification of pregnant women at risk of preeclampsia worsening could help to anticipated therapeutic intervention and improvement of the clinical outcome.
Aim: The ROADMAP-Early Onset Preeclampsia study (ROADMAP-EOP) aiming to respond to this unmet need assessed the clinical relevance of biomarkers of hypercoagulability and endothelial activation for prompt identification of pregnant women at high risk of preeclampsia.
Materials and Methods: An observational single center retrospective case-control study was conducted in pregnant women diagnosed with preeclampsia from July 2020 to July 2021. Pregnant women were retrospectively enrolled upon EOP diagnosis and stratified in mild EOP group (n=34) and severe EOP group (n=15 according to the criteria of the International Society for the Study of Hypertension in Pregnancy ). Eligible women were included in the study before initiation of any treatment for preeclampsia. The control group (n=35) consisted of women with uncomplicated pregnancy. All women were assessed with thromboelastometry, Calibrated Automated Thrombo-gram®, tissue factor activity (TFa), Procoagulant phospholipid dependent clotting time (Procoag-PPL), Proteins S, TFPI, D-dimer, antithrombin, thrombomodulin, fibrinogen prothrombin time and activated partial thromboplastin time. Primary and secondary end points were severe EOP and EOP respectively. Principal component analysis (PCA) was performed to check for associations among the biomarkers and the clinical out-come.
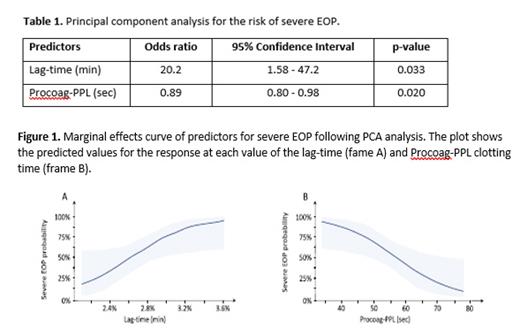
Results: There were no significant differences regarding age, gravidity, gestational age and the body mass index (BMI) between EOP groups and normal pregnancy as well as within the EOP groups. Platelet counts were similar in healthy pregnant women, and mild EOP group, and significantly lower in severe EOP (p<0.05). No other statistically significant differences were found between-groups. The PCA showed that the repartition of data allowed to discriminate the patients with EOP from healthy pregnant women as well as patients with mild EPO from those with severe EOP. When biomarkers of hypercoagulability were considered according to the severity of EOP in PCA analysis the lag-time and PPL clotting time were independently associated with the risk of severe EOP (Table 1and Figure 1)). The increase of the lag-time by 1 min was associated with a 20.2-fold increase of the risk of severe EOP. The marginal effects curve shows that for a lag-time longer than 2.6 min, the probability of severe EOP was higher than 50% .Longer Procoag-PPL clotting time was associated with lower risk of severe EOP. The marginal effects curve, showed that for Procoag-PPL clotting time longer than 58.75 sec the probability of severe EOP decreased by 50%. These data suggest that a biological score composed of the Procoag-PPL® clotting time and thrombin generation (Throbmogram-Thrombinoscope® PPP-reagent 1 pM-TF as-say) could be a useful tool to identify pregnant women with high probability of EOP
Conclusion: the Procoag-PPL® clotting time and thrombin generation assay reflect cellular derived hypercoagulability and allow accurate screening of pregnant women for the prompt identification of those at risk of EOP and the evaluation of its severity. This concept has to be validated in a large prospective cohort trial. . An external validation of the proposed methodology needs to be performed in a large prospective observational study.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal